Monoammonium phosphate (MAP) is a widely used source of phosphorus (P) and nitrogen (N).* It’s made of two constituents common in the fertilizer industry and contains the most phosphorus of any common solid fertilizer.
Production
MAP’s manufacturing process is relatively simple. While there are multiple methods, two common ways include:
A one-to-one ratio of ammonia (NH₃) and phosphoric acid (H₃PO₄) is reacted and the resulting slurry of MAP is solidified in a granulator
Introducing the two starting materials in a pipe-cross reactor, where the reaction generates heat to evaporate water and solidify MAP
An advantage of manufactured MAP is that lower-quality H₃PO₄ can be used compared with other P fertilizers often requiring a more pure grade of acid. The phosphorus pentoxide (P₂O₅) equivalent content of MAP varies from 48 to 61 percent, depending on the amount of impurity in the acid. The most common fertilizer composition is 11-52-0.
Chemical properties
- Chemical formula: NH₄H₂PO₄
- P₂O₅ range: 48-61%
- N range: 10-12%
- Water solubility (20 degrees): 370 g/L
- Solution pH: 4 to 4.5
Agricultural use
MAP has been an important granular fertilizer for many years. It’s water-soluble and dissolves rapidly in adequately moist soil. Upon dissolution, the two basic components of the fertilizer separate again to release ammonium (NH₄⁺) and phosphate (H₂PO₄⁻), both of which plants rely on for healthy, sustained growth.
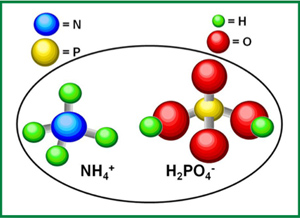
The pH of the solution surrounding the granule is moderately acidic, making MAP an especially desirable fertilizer in neutral- and high-pH soils. Agronomic studies show that, under most conditions, no significant difference exists in P nutrition between various commercial P fertilizers under most conditions.
Growers apply granular MAP in concentrated bands beneath the soil surface in proximity of growing roots or in surface bands. It’s also commonly applied by spreading across the field and mixing into the surface soil via tillage.
In powdered form, MAP is an important component of suspension fertilizers. When MAP is made with especially pure H₃PO₄, it readily dissolves into a clear solution dispersed as a foliar spray or added to irrigation water. The P₂O₅ equivalent content of high-purity MAP is usually 61 percent.
Management practices
No special precautions are needed with the use of MAP. The slight acidity associated with this fertilizer reduces the potential for NH₃ loss to the air. MAP can be placed in close proximity to germinating seeds without concern for NH₃ damage. However, MAP used in foliar spray or added to irrigation water, shouldn’t be mixed with calcium or magnesium fertilizers.
MAP has good storage and handling properties. Some of the chemical impurities (such as iron and aluminum) naturally serve as a conditioner to prevent caking. Highly pure MAP may have a conditioner added or may require extra care in handling to prevent clumping and caking.
As with all P fertilizers, employ appropriate management practices to minimize any nutrient loss to surface or drainage water.
A high purity source of MAP is used as a feed ingredient for animals. The NH₄⁺ is synthesized into protein and the H₂PO₄⁻ supports a variety of metabolic functions in animals.
Learn more about how management and practices support Advanced Crop Nutrition.
Non-agricultural uses
MAP is used in dry chemical fire extinguishers commonly found in offices, schools and homes. The extinguisher spray disperses finely powdered MAP, which coats the fuel and rapidly smothers the flame.
Discover why MicroEssentials® beats MAP/DAP commodity.
*MAP is also known as ammonium phosphate monobasic and ammonium dihydrogen phosphate
Source: Nutrient Source Specifics (No. 9), International Plant Nutrition Institute.