Liquid fertilizer solutions and fluid fertilizers are popular in many areas because they’re safe to handle, convenient to mix with other nutrients and chemicals, and are easily applied. A solution of urea [CO(NH₂)₂] and ammonium nitrate [NH₄NO₃] containing between 28 and 32 percent nitrogen (N) is the most popular fluid N fertilizer.
Production
Liquid urea-ammonium nitrate (UAN) fertilizer is relatively simple to produce. A heated solution containing dissolved urea is mixed with a heated solution of ammonium nitrate to make a clear liquid fertilizer. Half of the total N comes from the urea solution and half from the ammonium nitrate solution. UAN is made in batches in some facilities or in a continual process in others. No emissions or waste products occur during mixing.
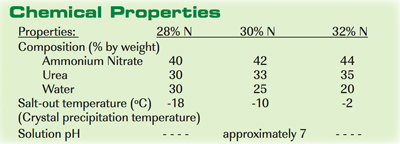
Since UAN is a concentrated N solution, its solubility increases as the temperature rises. To prevent the N components from precipitating as crystals, manufacturers make the UAN solutions more dilute in regions with cold winter temperatures. Therefore, the N concentration in commercial UAN fertilizers will vary from 28 to 32 percent, depending on geography. A corrosion inhibitor is usually added to the final solution to protect the steel in storage tanks.
Agricultural use
Solutions of UAN are widely used as a source of N for plant nutrition. The NO₃- portion (25 percent of the total N) is immediately available for plant uptake. The NH₄+ fraction (25 percent of the total N) can also be assimilated directly by most plants, but is rapidly oxidized by soil bacteria to form NO₃- (nitrate). Soil enzymes hydrolyze the remaining urea portion (50 percent of the total N) to form NH₄+, which subsequently transforms to NO₃- in most soil conditions.
Solutions of UAN are extremely versatile as a source of plant nutrition. Its chemical properties, make UAN compatible with many other nutrients and agricultural chemicals, so its frequently mixed with solutions containing phosphorus, potassium and other essential plant nutrients. Fluid fertilizers can be blended to precisely meet the specific needs of a soil or crop.
UAN solutions are commonly injected into the soil beneath the surface, sprayed onto the soil surface, dribbled as a band onto the surface, added to irrigation water, or sprayed onto plant leaves as a source of foliar nutrition. However, UAN may damage foliage if it’s sprayed directly on some plants, so dilution with water may be needed.
Management practices
Though UAN makes an excellent source of N nutrition for plants, since half of the total N is present as urea, extra management may be required to avoid volatile losses. When UAN remains on the surface of the soil for extended periods (a few days), soil enzymes will convert the urea to ammonium (NH₄+), a portion of which can evaporate as ammonia gas. Therefore, to avoid significant loss, UAN should not remain on the soil surface for more than several days. Inhibitors that slow these N transformations are sometimes added. When UAN is first applied to soil, the urea and the NO₃- molecules will move freely with water in the soil. The NH₄+ will be retained in the soil where it first contacts cation exchange sites on clay or organic matter. Within two to 10 days, most of the urea will be converted to NH₄+ and no longer be mobile. The originally added NH₄+ plus the NH₄+ coming from urea will eventually be converted to NO₃- by soil microorganisms.
Source: Nutrient Source Specifics, No. 7, International Plant
Nutrition Institute (IPNI)