Gypsum is a common mineral obtained from surface and underground deposits. It can be a valuable source of both calcium (Ca) and sulfur (S) for plants and can benefit certain soil properties under specific conditions.
Production
Gypsum is found in both crystal and rock forms. It generally results from the evaporation of saline water and is one of the more common minerals in sedimentary conditions. The white or gray-colored rocks are mined from open-pit or underground deposits, then crushed, screened, and used for a variety of purposes without further processing. Agricultural gypsum generally consists of CaSO₄·2H₂O (dihydrate). Under geological conditions of high temperature and pressure, gypsum is converted to anhydrite (CaSO₄ with no water).
Byproduct gypsum comes from fossil-fuel power stations where S is scrubbed from exhaust gas. Gypsum is also a byproduct from processing phosphate rock into phosphoric acid. Gypsum from recycled wallboard is finely ground and applied to soil as fertilizer.
Chemical properties
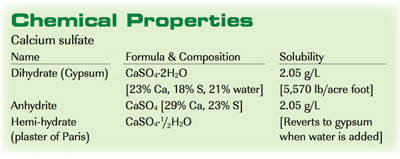
Agricultural use
Farmers typically add gypsum (sometimes called land plaster) to soils either to nourish plants or modify and improve soil properties. Gypsum is somewhat soluble in water, but more than 100 times more soluble than limestone in neutral pH soils. When applied to soil, its solubility depends on several factors, including particle size, soil moisture and soil properties. Gypsum dissolves in water to release Ca²⁺ and SO₄²⁻, with no significant direct impact on soil pH. In contrast, limestone will neutralize acidity in low-pH soils. In regions with acid subsoils, growers sometimes use it as a relatively soluble source of Ca for alleviation of aluminum toxicity.

Some soils benefit from application of gypsum as a source of Ca. In soils with excess sodium (Na), the Ca released from gypsum will tend to bind with greater affinity than Na on soil exchange sites, thus releasing the Na to be leached from the root zone. Where gypsum is used to remediate high-Na soils, it generally results in the enhancement of soil physical properties — such as reducing bulk density, increasing permeability and water infiltration, and decreasing soil crusting. In most conditions, adding gypsum by itself will not loosen compacted or heavy clay soils.
Source: Nutrient Source Specifics (No. 16), International Plant Nutrition Institute.