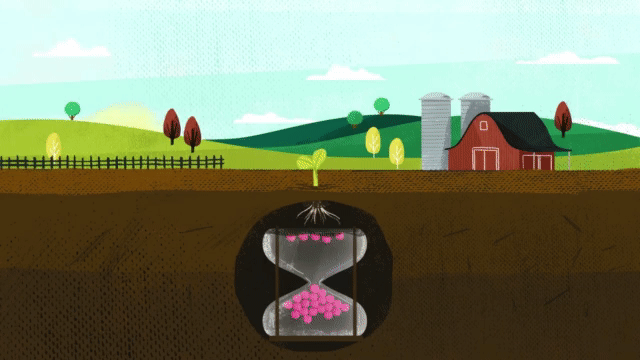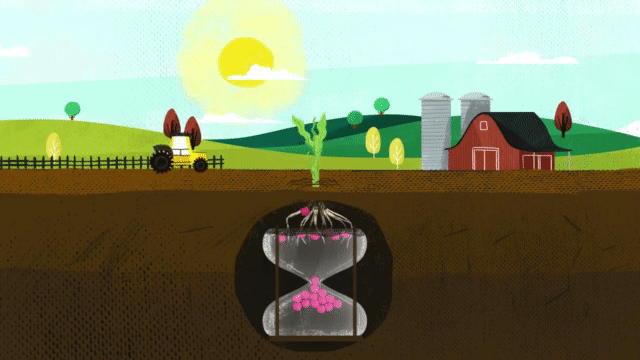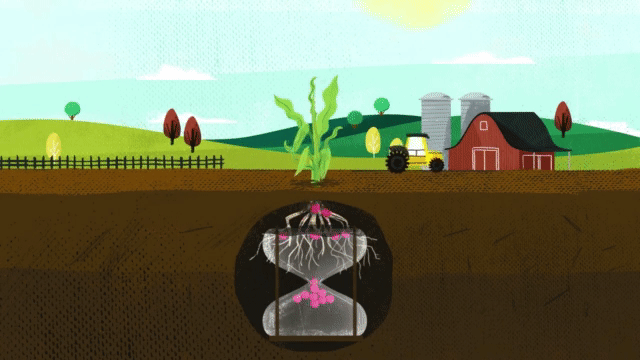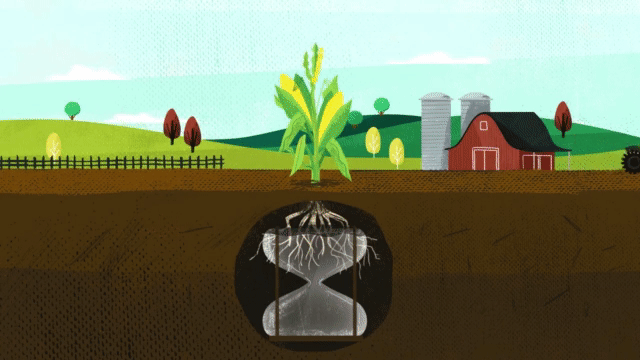
Corn takes up 38 percent of sulfur during the last three stages of growth. When deciding on a fertilizer application, it is critical that the fertilizer contains the nutrients necessary for optimum plant growth, including season-long sulfur.
Sulfur (S) is one of the 17 elements essential for plant growth, and the fourth most important after nitrogen (N), phosphorus (P) and potassium (K) in terms of amount required by corn. The two forms of S crops need to thrive are sulfate S (SO₄²⁻) and elemental S (S⁰). Sulfate sulfur is available immediately to the crop while S⁰ oxidizes for late-season sulfur uptake by the plants.
Plants can absorb S only through their root systems in the SO₄²⁻ form. Small amounts of sulfur dioxide gas can be absorbed through leaves, but this is of little consequence in the overall S nutrition of plants. This means that all soil S must be converted to SO₄²⁻ in order to be utilized by plants.
Elemental S is the most concentrated form of S; hence, it has lower transport costs and less of a dilution effect on other nutrients in fertilizers compared to sulfate-based fertilizers. Oxidation of elemental S is a critical step to get the plant-available S form all season long, and is affected by soil, environmental and fertilizer factors.
Key factors affecting elemental S oxidation
Oxidation of elemental S in soil is a microbial process requiring the presence of both water and oxygen (Fig. 1). The converted sulfate can be taken up by crops, used by microorganisms in respiration processes, or leached below the root zone in coarse-textured soils or high-rainfall areas. There is a wide variety of microorganisms in soil that are capable of oxidizing elemental S, including both bacteria and fungi; oxidation is not solely dependent on the presence of specific S-oxidizing organisms. Since the oxidation of S⁰ to SO₄²⁻ is a biological process conditions must favor the growth of the organisms in order for oxidation to proceed at optimum rate.
Figure 1: The reaction of elemental S (S⁰) oxidation in soils to sulfate (S₄²⁻).

Soil and environmental factors affecting elemental S oxidation
Oxidation is more rapid in warm, moist soils with high organic matter (OM) contents. Oxidation reactions of elemental S are also faster in alkaline soils than in acidic soils (Fig. 2). Of the soil and environmental factors affecting oxidation rate, temperature and Soil pH have the greatest effect.

Figure 2: Effect of soil pH on oxidation of elemental S across 10 soils.
Fertilizer attributes affecting elemental S oxidation
Oxidation of elemental S is also affected by the characteristics of the fertilizer, including the particle size of elemental S and/or its concentration in the fertilizer.
Oxidation is a surface-based process, and surface area increases dramatically as particle size decreases; therefore, particle size is one of the most important attributes affecting oxidation. When elemental S is dispersed through the soil, the oxidation of elemental S is faster as the particle size of the elemental S decreases (Fig. 3). In co-granulated elemental S fertilizers (i.e., where elemental S particles are co-granulated with macronutrients [N, P, K]), the oxidation is reduced compared to the elemental S particles of the same size dispersed through soil. This is not because the macronutrients reduce the oxidation rate, but because of the reduction in surface area of elemental S available for oxidation when dispersed in soil. Therefore, the lower oxidation rate of co-granulated elemental S can be explained by a reduction in the surface area of S in contact with the soil.

Figure 3: Effect of elemental S (S⁰) particle size on the rate of oxidation in soil where particles were dispersed throughout the soil.
The surface area available for oxidation for a given amount of S also depends on the concentration of elemental S in the granule due to decreased contact with the soil (Fig. 4). An Excel-based model has been produced by the Mosaic Fertilizer Technology Research Centre that integrates all soil, environmental and fertilizer granule factors that affect the oxidation of elemental S in soils to allow predictions of oxidation rates in various locations with defined fertilizer types. An example of model output is shown in Fig. 5. The oxidation of the S pastilles is predicted to be much slower than for the S in the MicroEssentials® granules. This is due to the much higher content of the S pastilles, resulting in less surface area exposed to the soil.

Figure 4: Schematic of dissolution of granulated fertilizers containing elemental S (S⁰), for a granule with (a) higher or (b) lower ES concentration

Figure 5: Effect of elemental S content of granule on oxidation for co-granulated products over two seasons.
MicroEssentials fertilizer ensures season-long sulfur availability by supplying both S₄²⁻ and S⁰ in the same granule. When MicroEssentials is applied to the soil, the sulfate sulfur is already in the proper form to feed the plant. Fertilizer granules rapidly dissolve and move sulfate-sulfur into the root zone for early-season development. Throughout the growing season, soil microbes convert the elemental sulfur into sulfate-sulfur for season-long feeding.
As mentioned earlier, the smaller the size of elemental sulfur particles, the easier it is for the microbial population to oxidize it into sulfate-sulfur. With other sulfur products that have larger particles, it may take multiple years for that elemental sulfur to break down and become available to the plant, whereas with MicroEssentials’ Fusion® technology, you get small, evenly distributed pieces of elemental sulfur that allow for mineralization throughout the growing season. It’s the best of both worlds. You get the sulfur that the plant needs early from the sulfate, and then the microbes oxidizing the elemental sulfur throughout the season provide the gradual feeding.
Talk to your local retailer about how MicroEssentials can fit into your crop-nutrient management plan to maximize your investment.