Diammonium phosphate (DAP) is the world’s most widely used phosphorus fertilizer. It’s made from two common constituents in the fertilizer industry, and its relatively high nutrient content and excellent physical properties make it a popular choice in farming and other industries.
Production
Ammonium phosphate fertilizers first became available in the 1960s, and DAP rapidly became the most popular in this class of products. It’s formulated in a controlled reaction of phosphoric acid with ammonia, where the hot slurry is then cooled, granulated and sieved. DAP handles and stores well. The standard nutrient grade of DAP is relatively high, at 18-46-0, so fertilizer products with lower nutrient content may not be labeled DAP.
The inputs required to produce one ton of DAP fertilizer are approximately 1.5 to 2 tons of phosphate rock, 0.4 tons of sulfur (S) to dissolve the rock, and 0.2 tons of ammonia. Changes in the supply or price of any of these inputs will impact DAP prices and availability. The high nutrient content of DAP helps reduce handling, freight and application costs. DAP is produced in many locations in the world and is a widely traded fertilizer commodity.
Chemical properties
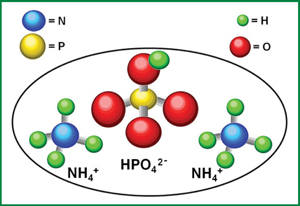
- Chemical formula: (NH₄)₂HPO₄
- Composition: 18% N, 46% P₂O₅ (20% P)
- Water solubility (20 degrees C): 588 g/L
- Solution pH: 7.5 to 8
Agricultural use
DAP fertilizer is an excellent source of P and nitrogen (N) for plant nutrition. It’s highly soluble and thus dissolves quickly in soil to release plant-available phosphate and ammonium. A notable property of DAP is the alkaline pH that develops around the dissolving granule.
As dissolving DAP granules release ammonium, the seedlings and plant roots nearest the volatile ammonia can be harmed. This potential damage more commonly occurs when the soil pH is greater than 7, a condition that often exists around the dissolving DAP granule. To prevent such damage, users should avoid placing high concentrations of DAP near germinating seeds.
The ammonium present in DAP is an excellent N source and will be gradually converted to nitrate by soil bacteria, resulting in a subsequent drop in pH. Therefore, the rise in soil pH surrounding DAP granules is a temporary effect. This initial rise in soil pH neighboring DAP can influence the micro-site reactions of phosphate and soil organic matter.
Management practices
Differences in the initial chemical reaction between various commercial P fertilizers in soil become minor over time (within weeks or months) and are minimal as far as plant nutrition is concerned. Most field comparisons between DAP and monoammonium phosphate (MAP) show only minor or no differences in plant growth and yield due to P source with proper management.
Learn more about how management and practices support Advanced Crop Nutrition.
Non-agricultural uses
DAP has additional uses, including:
- As a fire retardant. For example, a mixture of DAP and other ingredients can be spread in advance of a fire to prevent a forest from burning. It then becomes a nutrient source after the danger of fire has passed.
- In various industrial processes, such as metal finishing
- An addition to wine to sustain yeast fermentation
- An addition to milk to produce cheese cultures
Discover why MicroEssentials® beats MAP/DAP commodity.
Source: Nutrient Source Specifics (No. 17), International Plant Nutrition Institute.